FDA Issues Class I Recall for Omnipod DASH Insulin
By Dave Quaile, MD /alert Contributor
December 15, 2022
A class I recall has been issued for the Omnipod DASH Insulin Management System’s personal diabetes manager (PDM) by the device’s manufacturer, according to a press release from the FDA.
The use of the Omnipod DASH insulin Management System may cause serious injury or death. The device was designed to deliver insulin at set and variable rates to manage diabetes for patients who require insulin.
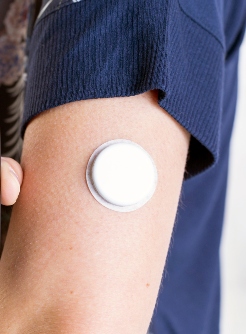
The PDM is a small, reusable handheld rechargeable battery-powered locked-down Android mobile device with features similar to a smartphone. The device is controlled exclusively using the PDM, and the two parts interact wirelessly using secure Bluetooth.
The device has been recalled due to battery issues including, battery swelling, battery fluid leakage, and extreme overheating that may become a fire hazard. A total of 455 complaints were filed with Insulet regarding the battery, including three fires. However, there were no reported injuries or death.
According to the manufacturer, current customers affected by the recall, including those treated with temporary PDMs, will be shipped updated devices in the coming months as they become available.
Patients and physicians with questions regarding the recall can visit the Insulet website or call 1-800-641-2049 to speak with the Insulet Customer Care team.
--
Disclosures: MD /alert could not confirm financial disclosures at the time of reporting.
Photo Credit: Getty Images.























.jpg)
.jpg)