CGM Bests Blood Glucose Monitoring Among Patients with Poorly Controlled Type 2 Diabetes
By Dave Quaile, MD /alert Contributor
December 22, 2021
Continuous glucose monitoring may be beneficial for patients with poorly controlled type 2 diabetes treated with basal insulin when compared to blood glucose meter monitoring, according to results published in JAMA.
According to the randomized clinical trial from Roy W. Beck, MD, PhD, from the Jaeb Center for Health Research Foundation, Inc, in Tampa Florida, adult patients with type 2 diabetes saw a greater decrease in HbA1c level over 8 months with continuous glucose monitoring (CGM) than with blood glucose meter monitoring (BGM).
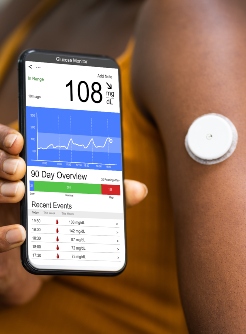
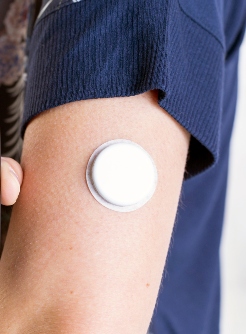
“Real-time continuous glucose monitoring, by providing glucose measurements as often as every 5 minutes, low and high glucose alerts, and glucose trend information, has the potential to better inform diabetes management decisions compared with episodic self-monitoring with a BGM,” Beck and colleagues wrote. “Studies have demonstrated that CGM improved glycemic control in individuals with type 1 diabetes and with type 2 diabetes using insulin regimens with basal plus prandial insulin. The role of CGM in individuals with type 2 diabetes using less-intensive insulin regimens is not well defined.”

To determine the efficacy of CGM in adults with type 2 diabetes treated with basal insulin without prandial insulin in primary care practices, Beck and colleagues designed a randomized clinical trial at 15 centers between July 2018 and October 2019.
Patients included in the study received diabetes care from a primary care physician and were treated with 1-2 daily injections of long-or intermediate-acting basal insulin without prandial insulin, with or without non-insulin glucose-lowering medications.
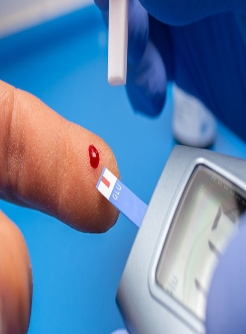
Patients were randomly assigned 2:1 to CGM (n = 116) or BGM (n = 59).
The primary outcome of the study was hemoglobin A1c (HbA1c) level at 8 months and secondary outcomes included CGM-measured time in target glucose range of 70 to 180 mg/dL, time with glucose level higher than 250 mg/dL and mean glucose level at 8 months.
The researchers reported that 165 of the 175 randomized patients (94%) completed the trial.
According to the results of the study, mean HbA1c level decreased from 9.1% to 8.0% at 8 months in the CGM group and from 9.0% to 8.4% in the BGM group (adjusted difference, −0.4%; 95% CI, −0.8 to −0.1; P = .02) at baseline.
In the CGM group, compared with the BGM group,
the mean percentage of CGM-measured time in the target glucose range of 70 to 180 mg/dL was 59% vs 43% (adjusted difference, 15%; 95% CI, 8-23; P < .001),
the mean percentage of time at greater than 250 mg/dL was 11% vs 27% (adjusted difference, −16%; 95% CI, −21 to −11]; P < .001), and
the mean glucose values were 179 mg/dL vs 206 mg/dL (adjusted difference, −26 mg/dL; 95% CI, −41 to −12]; P < .001).
One participant (1%) in the CGM group and one participant (2%) in the BGM group experienced severe hypoglycemic events.
“In this randomized trial of patients with type 2 diabetes and poor glycemic control treated with basal insulin without prandial insulin and recruited from a primary care setting, HbA1c level improvement at 8 months was significantly greater in participants using CGM than in participants using a BGM alone for glucose monitoring,” the researchers wrote.
According to an accompanying editorial from Monica E. Peek, MD, MPH, MS, from the Section of General Internal Medicine at the Chicago Center for Diabetes Translation Research and the Center for the Study of Race, Politics and Culture at the University of Chicago, and Celeste C. Thomas, MD, MS, from the Section of Endocrinology at the Chicago Center for Diabetes Translation Research at the University of Chicago, the studies by Karter et al and Martens et al provide additional evidence that patients with type 2 diabetes benefit from the use of CGM in terms of improved HbA1c level, time spent in the target blood glucose range, and reduced hypoglycemic episodes.
Peek and Thomas point out that the benefit from CGM may be primarily due to patient factors, such as insulin adherence and lifestyle modifications, and provide a powerful narrative that CGM may be a useful technology that helps control diabetes among multiple patient groups.
“Important policy changes in Medicare eligibility to CGM for type 2 diabetes and institutional changes that promote its use in primary care will go a long way to improving diabetes control and reducing complications, particularly among the populations most in need,” they wrote. “The time has come to broaden access to CGM for patients with type 2 diabetes.”
Disclosure: Beck reports receiving grant funding and study supplies from Bionics and Tandem Diabetes Care, study supplies from Ascencia, Medtronic and Roche, consulting fees and study supplies from Eli Lilly and Novo Nordisk and consulting fees from Bigfoot Biomedical, Diasome, Insulet and vTv Therapeutics. Peek reports receiving speaking fees from PRIME Education. Thomas reports no relevant financial disclosures. Please see the study for a full list of author disclosures.
Photo Credit: Getty Images























.jpg)
.jpg)